1. MANUSCRIPT FORMATTING
Manuscript format must be in accordance with the ICMJE-Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals (updated in December 2015 - http://www.icmje.org/icmje-recommendations.pdf)Papers that do not comply with the format of the Journal will be returned to the author for correction without further review. Therefore, to avoid loss of time and work, authors must carefully review the submission rules.
1.1 General Format
The manuscript should be typed in a Microsoft Word™ file, single-column format, double-spaced with 2.5 cm margins on each side, and 11-point type in Times New Roman font.
All abbreviations in the text must be defined the first time they are used (both in the abstract and the main text), and the abbreviations should be displayed in parentheses after the definition. Abbreviations should be limited to those defined in the AMA Manual of Style, current edition. Authors should avoid abbreviations in the title and abstract and limit their use in the main text.
Decimal points should be used in decimals throughout the manuscript. Measurements should be reported using the metric system according to the International System of Units (SI). Consult the SI Unit Conversion Guide, New England Journal of Medicine Books, 1992. An extensive list of conversion factors can be found at http://www.unc.edu/~rowlett/units/. For more detail, see http://www.amamanualofstyle.com/oso/ public/jama/si_conversion_table.html. When a drug, product, hardware, or software mentioned within the main text product information, including the name of the product, producer of the product, city of the company and the country of the company should be provided in parenthesis in the following format: “Discovery St PET/CT scanner (General Electric, Milwaukee, WI, USA)”.
1.2 Article Type
Identification of article type is the first step of manuscript submission because article type dictates the guidelines that should be used (see below, Using Guidelines), including formatting and word limits of the manuscript. Balkan Medical Journal does not accept case reports. The main categories of article type are outlined below:
Original Article: Original contributions are manuscripts containing substantial novel research. These articles can include randomized controlled trials, observational (cohort, case-control or cross-sectional) studies, descriptive studies, diagnostic accuracy studies, systematic reviews and meta-analyses, nonrandomized behavioral and public health intervention trials, experimental animal trials, or any other clinical or experimental studies. Abstracts should not exceed 500 words and should be structured with the following subheadings: Background, Aims, Study Design (case control study, cross sectional study, cohort study, randomized controlled trial, diagnostic accuracy study, meta-analysis and systemic review, animal experimentation, non-randomized study in behavioral sciences and public health, etc.), Methods, Results, and Conclusion. The main text should be structured with the following subheadings: Introduction, Material and Methods, Results, Discussion, Acknowledgments, References, Tables, and Figure Legends. The main text should not exceed 4000 words, excluding the abstract, references, tables, and figure legends. There should be a maximum of 50 references. All papers but cell studies require ethical approval.
Brief Reports: Brief reports are short and peer-reviewed articles including small case series, negative trials, the preliminary results and others that are not to be published as a full text paper. The main text should be less than 2000 words with a maximum 20 references and have no more than 3 display items (Tables or figures). Abstracts should not exceed 250 words and should be structured with the following subheadings: Aims, Methods, Results, and Conclusion. All papers require ethical approval.
Invited Review Article: Invited review articles are comprehensive analyses of specific topics in medicine, which are written upon invitation due to extensive experience and publications of authors on the review subjects. All invited review articles will also undergo peer review prior to acceptance. Review articles must not exceed 5000 words for the main text (excluding references, tables, and figure legends) and 500 words for the abstract. A review article can be signed by no more than 7 authors and can have no more than 150 references.
Case Report: Balkan Medical Journal does not accept case report type of manuscripts. Authors are welcomed to submit their cases in a Letter to the Editor or Clinical Image format. For the instructions for Letter to the Editor and Clinical Image, please see below.
Clinical Image: For educational purposes, the journal publishes original, interesting, and high quality clinical images having a brief explanation (maximum 700 words excluding references but including figure legends) and of educational significance. The figure legend should contain no more than 100 words. It can be signed by no more than 5 authors and can have no more than 10 references and 1 figure and/or table. Any information that might identify the patient or hospital, including the date, should be removed from the image. An abstract is not required with this type of manuscripts. The main text of clinical images should be structured with the following subheadings: Case, and References. All papers require the patient's consent.
Letter to the Editor: A Letter to the Editor is a type of manuscript that discusses important or overlooked aspects of a previously published article. Letters in reference to a journal article must not exceed 700 words (excluding references).An abstract is not required with this type of manuscripts. A letter can be signed by no more than 4 authors and can have no more than 10 references and 1 figure and/or table. Authors might be asked for a reply to the letter. Replies must be submitted through submission system as well.
Scientific Letters: Scientific letters are informative documents describing the progress and findings of a research project. They should provide a summary and key details of the methods used in the research. The scienrific letter should end with conclusions that are supported by the research and recommendations for future studies. Scientific letters in reference to a journal article must not exceed 1250 words (excluding references). An abstract is not required with this type of manuscripts. A letter can be signed by no more than 5 authors and can have no more than 15 references and 1 figure and/or table.
Editorial Comment: Editorial comments are a brief remark on an article published in the journal by the reviewer of the article or by a relevant authority. Most comments are invited by the Editor-in-Chief but spontaneous comments are welcome. It must not exceed 1250 words (excluding references). An abstract is not required with this type of manuscripts. It can have no more than 15 references and 1 figure or table.
Health Policy: Balkan Medical Journal encourages the submission of health policy articles formatted as Original Research and Review Articles. Submissions should aim to enhance readers’understanding of the impact of health policy on patients, medical institutions, or populations. The main text should not exceed 3000 words, excluding the abstract, references, tables, and figure legends. There should be a maximum of 50 references, 4 tables, and/or figures.
Other: Editorials, editorial comments, book reviews, and reports on publication and research ethics are requested by the Editorial Board.
| Type of Manuscript | Word Limit | Abstract Word Limit | Reference Limit | Author Limit |
| Original Article | 40001 | 5004 | 50 | None |
| Brief Reports | 20001 | 300 | 30 | None |
| Invited Review | 50001 | 500 | 150 | 7 |
| Clinical Image | 7002 | N/A | 10 | 5 |
| Letter to Editor | 7003 | N/A | 10 | 4 |
| Scientific Letter | 1250 | N/A | 15 | 5 |
| Editorial Comment | 1250 | N/A | 15 | 4 |
| Consensus Report | 9000 | 500 | 300 | None |
1This should not include the abstract, references, tables or figure legends.;
2This should include the figure legends.
3This should not include the references.
4Should be structured with the following subheadings: Background, Aims, Study Design, Methods, Results, and Conclusion.
5Should be structured with the following subheadings: Background and Conclusion.
6If a reasonable explanation exists, the Editorial Board may accept not to limit the number of authors.
7It is possible to make exceptions to the rules if the Editorial Board determines it is necessary.
1.3. Clinical Trials and Reporting Guidelines
The Balkan Medical Journal encourages the registration of all clinical trials via ClinicalTrials.gov (www.clinicaltrials.gov) or one of the registries of the WHO’s International Clinical Trials Registry Platform (ICTRP: http://www.who.int/ictrp/network/primary/en/index.html). Especially the phase 3 clinical trials must be registered at or before the time of first patient enrollment. The name of the registry and the registration number together with the information of funding source should be provided at the end of the abstract.
Authors should also refer to the guidelines below when preparing their manuscript
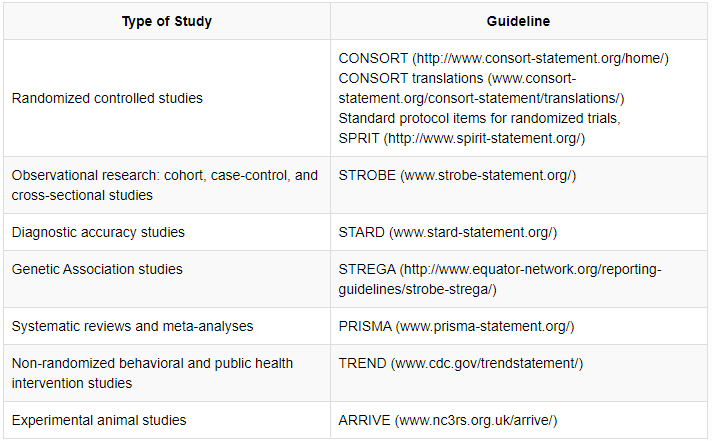
For further information on the reporting guidelines for health research, authors are suggested to refer to the EQUATOR network website (http://www.equator-network.org/)
1.4. Data Sharing: Please see the editorial policy.
1.5. Reporting Sex/Gender: Please see the editorial policy.
1.6. Preprint: Please see the editorial policy.
2. PREPARATION AND SUBMISSION OF A MANUSCRIPT
The entire submission process for a manuscript is completed online through the self-explanatory online submission system through the following website: https://balkanmedj.manuscriptmanager.net. Please be informed that we will ask you to suggest two potential reviewers during the manuscript submission process. The submission should be divided into SEPARATE files in the following order:
2.1. Cover Letter
2.2. Title Page
2.3. Main Document (Main text, references, tables, and figure legends. Abstract of the manuscript should be submitted through the submission system)
2.4. Figures
2.5. The ICMJE Conflict of Interest form and Data-sharing Statement form
2.1. Cover Letter
The cover letter should include the article title, article type, and the full name of the corresponding author. The corresponding author should briefly summarize why his/her paper is a valuable addition to the scientific literature and specify the type of article he/she is submitting (for example, original article, review article, clinical image). The cover letter should also include a statement declaring the absence or presence of a conflict of interest (please refer to the ICMJE Conflict of Interest formpage for details). Furthermore, there should be a statement that the manuscript has not already been published, accepted or under simultaneous review for publication elsewhere. Balkan Medical Journal does not accept multiple submission and duplicate submission even though the previous one was published in a different language. Please refer to the editorial (Balkan Med J 2013;30:1-2) ICMJE recommendations (http://www.icmje.org/recommendations/browse/publishing-and-editorial-issues/overlapping-publications.html) on this subject for more details. For manuscripts that have been presented orally or as a poster, this must be stated on the title page with the date and the place of the presentation. An example of a cover letter can be found on the journal’s webpage."
2.2. Title Page
This should include:
The complete manuscript title
The running head
Word counts for the abstract and text (the text word count does not include references, tables, and figure legends)
The number of references, and the number of figures and tables
All authors' full names with ORCIDs, affiliations and e-mail addresses (all authors should meet the ICMJE’s requirements for authorship – see details at “Copyright Transfer and Acknowledgement of Authorship Form”
The name, address, telephone and fax numbers and email address of the corresponding author
Keywords (3-6 key words from the list provided in Index Medicus under “Medical Subject Heading (MeSH)”
Information about where and when the study has previously been presented.
Acknowledgements: All contributors who do not meet the criteria for authorship (ICMJE: authorship and contributorship) and the statement of conflict of interest and funding should be declared under this subheading.
Note: Information pertaining to the author to be published on the title page should be uploaded separately in the system. The relevant details will be reflected on the title page during the publishing process.
Abstracts
Original articles and invited review articles should include an abstract. Abstracts for original articles should be structured with the following subheadings: Background, Aims, Study design, Methods, Results, and Conclusion. Abstracts for review articles should not be structured. Clinical images, Editorials, Letters to the Editor, and Commentaries should not contain an abstract. Please find the guidelines for abstracts of specific types of manuscripts under the Main Text section below.
2.3. Main Document
The main document should include the main text, the reference list, tables, and figure legends, respectively.
Any information that may indicate an individual or institution should be excluded from the main document to ensure a blinded review process.
References
Authors are encouraged to cite primary literature rather than review articles in order to give credit to those who have performed the original work. Reference listings must be in accordance with ICMJE standards and numbered consecutively at the end of the manuscript in the order in which they are mentioned in the text. While citing publications, preference should be given to the latest, most up to date publications. If an ahead of print publication is being cited the DOI number should be provided. Authors are responsible for the accuracy of references. Journal titles should be abbreviated in accordance with the journal abbreviations in Index Medicus/ Medline/PubMed (for journal abbreviations consult the List of Journals indexed for MEDLINE, published annually by NLM). The name of the journal should be written in italic, and a point should be placed at the end. If the authors' names are more than 6, the first 3 authors should be written and should be followed by "et al.". Page numbers should be written openly. In the main text of the manuscript, references should be cited using Arabic numbers in parentheses. The reference styles for different types of publications are presented in the following examples:
Journal article: Korytina G, Kochetova O, Akhmadishina L, Viktorova E, Victorova T. Polymorphisms of cytochrome P450 genes in three ethnic groups from Russia. Balkan Med J. 2012;29:252-260.
Book: Tos M. Cartilage tympanoplasty. 1st ed. Stuttgart-New York: Georg Thieme Verlag; 2009.
Book chapter: Tos M, Stangerup SE. The relationship between secretory otitis and cholesteatoma. In: Tos M, Thomsen J, Peitersen E, editors. Cholesteatoma and mastoid surgery. Amsterdam: Kugler & Ghedini; 1989:325-330.
Abstract: Gurakar A, Elsahwi K, Akdogan M, Wright H, Nour, B, Sebastian T, et al. Asplenia and primary sclerosing cholangitis (PSC): A mere coincidence? Hepatology. 2002;36:673a (abstract).
Article in electronic format: Morse SS. Factors in the emergence of infectious diseases. Emerg Infect Dis (serial online) 1995 Jan-Mar (cited 1996 June 5): 1(1): (24 screens). Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2626828/pdf/8903148.pdf.
For other reference style, please refer to “ICMJE Uniform Requirements for Manuscripts Submitted to Biomedical Journals: Sample References”
Tables
Tables should be presented within the main document after the reference list. All tables should be referred to within the main text and they should be numbered consecutively in the order they are referred to within the main text. A descriptive title should be provided for all tables and the titles should be placed above the tables. Abbreviations used in the tables should be defined below the tables (even if they are defined within the main text). Tables should be created using the “insert table” command of the word processing software and they should be arranged clearly to provide an easy reading.
2.4. Figures and Figure Legends
Figures, graphics and photographs should be submitted as separate files (in TIFF or JPEG format) through the submission system. They should not be embedded in a Word document. When there are figure subunits, the subunits should not be merged to form a single image. Each subunit should be submitted separately through the submission system. Images should not be labelled (a, b, c, etc.) to indicate figure subunits. Thick and thin arrows, arrowheads, stars, asterisks and similar marks can be used on the images to support figure legends. Like the rest of the submission, the figures too should be blind. Any information within the images that may indicate an individual or institution should be blinded. The minimum resolution of each submitted figure should be 300DPI. To prevent delays in the evaluation process all submitted figures should be clear in resolution and large in size (minimum dimensions 100x100 mm)
Figure legends should be listed at the end of the main document. When there are figure subunits, the figure legends should be structured in the following format.
Example: Figure 1. a-c. Primary culture of choroid plexuses on day 2 after seeding of dissociated cells (×400). Nestin staining in green (a). GFAP staining in red (b). Nuclear labelling in blue and merged images (c)
2.4.1. Graphical Abstracts
We believe sharing your valuable work will be important for our journal and your academic career. When you submit your article, you must also upload your graphical abstract work to the system.
Your graphical abstract should describe your article with tables and figures or essential statistical data. We aim to convert the content corresponding to the images into graphics as symbols.
You can find the samples of graphical abstracts below.
2.5. COPYRIGHT AGREEMENT AND ACKNOWLEDGEMENT OF AUTHORSHIP FORM
This is a statement of scientific contributions and responsibilities of all authors. The form is available for download at the journal’s webpage here.
The ICMJE recommends that authorship be based on the following 4 criteria:
♦Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND
♦Drafting the work or revising it critically for important intellectual content; AND
♦Final approval of the version to be published; AND
♦Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.”
A contributor should meet all four criteria to be identified as an author. If a contributor does not meet all four criteria he/she should be acknowledged in the acknowledgements section of the manuscript.
For more details please refer to the ICMJE’s definition of the role of authors and contributors at http://www.icmje.org/recommendations/browse/roles-and-responsibilities/defining-the-role-of-authors-and-contributors.html
2.6. The ICMJE Conflict of interest form and Data-sharing Statement form
During submission, the ICMJE Conflict of Interest form should be filled, saved to your computer and submitted to the Balkan Medical Journal together with your manuscript. Please refer to “conflict of interest policy” for more information. Balkan Medical Journal require authors to submit a Data-sharing Statement form for clinical trials and register a data-sharing plan when registering a clinical trial on or after Jan 1, 2019. Please refer to “data-sharing policy” for more information.
Creative Commons License Agreement Form
After the acceptance of the articles, the corresponding author must sign the Creative Commons License Agreement form and send it to the publisher. Please refer to “open access policy” for more information.
3. CHECKLIST
To finalize submission, the corresponding author should ensure that all files mentioned below have been uploaded:
♦ A cover letter containing;
the article title and type and the full name of the corresponding author
a brief statement describing the novelty and importance of the work
a statement declaring the absence or presence of a conflict of interest, ethics approval and/or patient consent for publication, the funding information, and the data availability a statement that the manuscript has not been previously published or accepted for publication and is not submitted or under simultaneous review for publication elsewhere
♦ A title page including;
Authors’ affiliations and e-mail addresses, including the name of the corresponding author
A statement of the date and place of the meeting where the manuscript was presented orally or as a poster, if necessary
Acknowledgements
♦ Structured abstract and text
♦ All Figures have been uploaded and appear correctly
♦ The author contribution and conflict of Interest statement (ICJME) forms
♦ Permission for reprinted figures, tables, materials or photographs has been obtained
When submitting a revised version of a paper, the author must submit a detailed “Response to reviewers” that states point by point how each issue raised by the reviewers has been covered and where it can be found (each reviewer's comment followed by the author’s reply and line numbers where the changes have been made) as well as an annotated copy of the main document.
Revised manuscripts must be submitted within 45 days from the date of the decision letter. If the revised version of the manuscript is not submitted within the allocated time, the revision option will be automatically cancelled by the submission system. If the submitting author(s) believe that additional time is required, they should request this extension before the initial 45-day period is over.
4. PROOFS AND DOI NUMBER
Manuscripts accepted for publication are provided with a DOI number immediately after acceptance. Accepted manuscripts are copy-edited for grammar, punctuation, and format by professional language editors. Once the publication process of a manuscript is completed it is published online on the journal’s webpage as an ahead of print publication before it is included in its scheduled issue. A PDF proof of the accepted manuscript is sent to the corresponding author and their publication approval is requested within 2 days of their receipt of the proof.
5. APPEALS AND COMPLAINTS
Please see the editorial policy.
6. DOCUMENTS TO DOWNLOAD
Cover Letter, Copyright Transfer and Acknowledgement of Authorship Form, Data-sharing Statement Form, The ICMJE Conflict of Interest Form
Editor- in- Chief: Prof. Servet ALTAY
Department of Cardiology, Trakya University Faculty of Medicine, Edirne, Türkiye.
Phone: +90 284 235 76 41/ 4217-4315 (internal)
E-mail: [email protected]
Editorial Office: Address: Balkan Medical Journal, Balkan Campus of Trakya University, Medical Faculty, 22030 Edirne, Türkiye.
Phone: +90 284 235 76 41/1430 (internal)
Fax: +90 284 235 76 52
E-mail: [email protected]